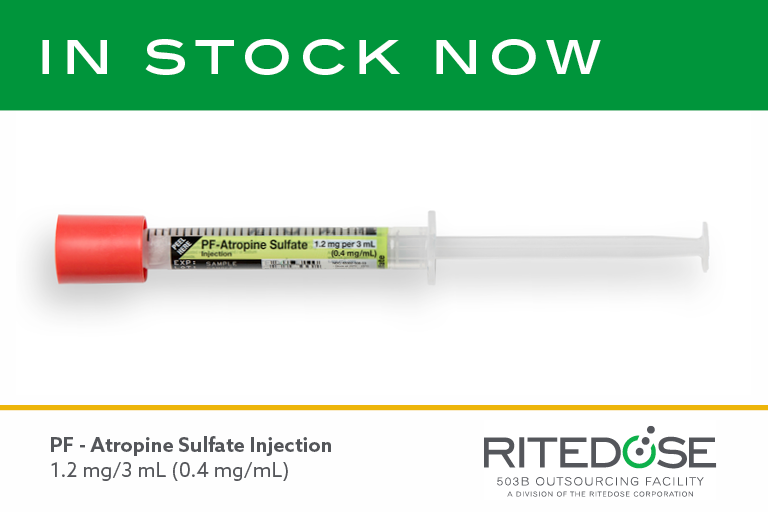
Ritedose Launches Critical Care Single-Dose Syringe Product Amid National Shortage: Atropine Sulfate Injection 1.2 mg/3 mL (0.4 mg/mL)
COLUMBIA, S.C. (September 10, 2021) – Ritedose, a 503B outsourcing facility located in South Carolina, just launched a new unit dose syringe product, Atropine Sulfate Injection 1.2 mg/3 mL (0.4 mg/mL). It is the last of a 12-product, six-month rollout…
READ MORE