WHO WE ARE
Working for the
greater good.
CORE VALUES
Five core values govern our daily business decisions, shape our company culture and ensure we deliver the highest value to all stakeholders, employees and customers.
See the real person we are working for and put their interests above all else.
Be accountable for our actions and collaborate openly and directly.
Put the time in to make it right and improve the process every day.
Operate with humility and gratitude, understanding it’s not about us.
Realize the impact of our work in the world and lend our time and resources to the communities we serve.

MISSION
VISION
RITEDOSE LEADERSHIP
Executive Leaders
Our forward-looking executive team is made up of dedicated, focused, and experienced leaders. Their combined knowledge and experience collaborate to make a difference in the markets we serve.
Chief Executive Officer

Jody Chastain
Chief Human Resources Officer

Janisha Thomas
Chief Information Officer

Shawn Miller
Chief Financial Officer

Duane Miskelly
EVP of Operations

Shawn Collins
EVP of Technical Services

Angie Koen
EVP of Quality

Mohammad Sadeghi
INNOVATION
We will never stop.
We are committed to continuous innovation for as long as patients need medications. Our talented team works diligently every day to enhance patients’ lives, utilizing data analysis and developing custom technology. Whether in early-phase clinical or at a commercial scale, we collaborate with our customers to provide ambitious, personalized solutions.

Patient-inspired.
Purpose driven.

Our patients
Patients are at the heart of everything we do. We honor the patients who rely on the high-quality medications we produce each day.

Our culture
A culture of excellence, led by our employees, means we support and empower each other, as well as the communities we serve.

Our community
We value our community. Our diverse company is committed to giving back and supporting the greater good for our patients and the world.
ESG STATEMENT
Our impact.

Healthy planet. Healthy society.
From the water, we drink to the air we breathe. Ritedose is committed to battling climate change and sustaining a healthy environment. We are determined to meet the Science Based Targets Initiative (SBTi) Net-Zero Standard. Because this is about a health-filled future for us all.

People-centered.
From patients, to employees, to clients, to our community, our passion is people. We strive every day to create a place where employees are happy and engaged and where we are an active partner with our communities and serve them well.
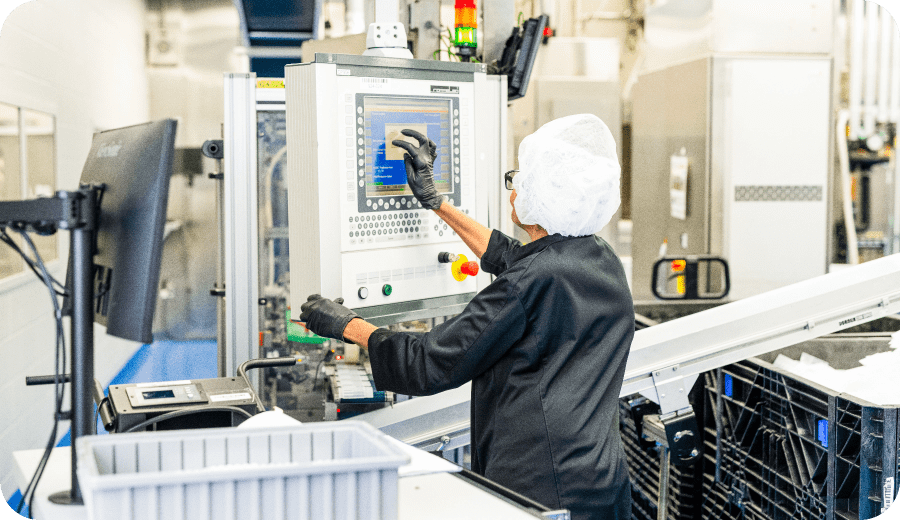
Quality products. Quality of life.
In every product we develop, every process we improve, and every drug we manufacture, we see our patients and their caregivers. That’s why Ritedose rigorously controls our supply chain, processes and outcomes to produce the safest delivery systems possible. Only the highest quality will do because our product is someone’s quality of life.
COMPLIANCE (AMP) Ritedose is considered outstanding for the safe manufacturing of generic and CDMO therapies, either meeting or exceeding FDA, USP and other regulatory and industry requirements. All our processes and procedures in our world-class cGMP facilities are carefully tested, audited and validated to ensure high-quality delivery of much-needed medicines. These tests include annual internal audits, supplier management and site audits, external audit management, product reviews and quarterly management reviews. We regularly pass inspections from regulatory and industry authorities without significant comments.
Say hello.
CONTACT US
Thank you for
your interest.
We look forward to connecting with you. We are excited about the opportunity to learn more about you and your needs.
